 A nationwide outbreak of fungal meningitis linked to contaminated steroid shots that so far has claimed 15 lives and sickened 205 in 14 states is shedding light on a largely unregulated part of the pharmaceutical industry.
A nationwide outbreak of fungal meningitis linked to contaminated steroid shots that so far has claimed 15 lives and sickened 205 in 14 states is shedding light on a largely unregulated part of the pharmaceutical industry.
“The recent outbreak of dangerous, potentially deadly disease indicates a clear and present need for stronger accountability and oversight,” Senator Richard Blumental said in a letter sent to the Food and Drug Administration (FDA).
On Tuesday, House and Senate lawmakers called for briefings on the outbreak that could lead to harsher regulations against compounding companies – facilities that mix drugs and package them into specific medications. Currently, only the drug ingredients are regulated by the FDA, not the specialized formulations.
“Stricter scrutiny by the FDA could help prevent contamination of medicines – apparently the cause of this 23-state crisis – produced by such compounding pharmacies,” Blumental said.
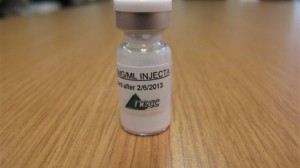
The mass meningitis outbreak is linked to tainted methylprednisolone acetate steroid injections manufactured by specialty Massachusetts pharmacy The New England Compounding Center and its sister pharmacy, Ameridose – the faulty drugs were shipped to 76 facilities in 23 states. The contaminated steroid shots were recalled on Sept. 26 and all other products produced by the compound pharmacies were pulled on Oct. 6.
Nearly 200 cases of the rare fungal infection have been reported in Illinois, Indiana, Texas, New Hampshire, Idaho, Minnesota, Michigan, Indiana, Ohio, Tennessee, Florida, North Carolina, Virginia, Maryland, and New Jersey since the initial recall, according to the Center for Disease Control and Prevention. It is estimated that around 14,000 patients were exposed to the tainted epidurals – the number of reported cases is expected to grow to around 650.
“We expect that most people who were exposed to this will not develop a fungal infection,” said Dr. David Reagan, chief medical officer for the Tennessee Department of Health.
Regardless, patients who received the injection after May 21 and experience swelling, weakness, headaches, nausea, dizziness, back pain or other related symptoms should get in touch with their health providers immediately – even mild symptoms are cause for alarm.
“Patients who feel ill and are concerned about whether they received a medication from one of the NECC products recalled on September 26 should contact their physician,” the CDC said in an advisory.
Unlike other, more common forms of meningitis, the fungal infection is not contagious.
Sources: CDC.gov, foxbusiness.com, foxnews.com, vitals.nbcnews.com, cbs.com, AP Photo



